STRAUMANN® EMDOGAIN®

For 30 years the world has changed but regeneration has always had one name: Emdogain®
Setting the gold standard in periodontal regeneration since 1995, emdogain has been at the forefront of periodontal regeneration, shaping the future of dentistry and transforming patient care.
Inspired by nature and powered by science, this unique gel harnesses the body’s natural healing process to restore both hard and soft tissues.
30 years Emdogain®
Emdogain - 30 years in the market
1,700 scientific publications (>600 clinical)2
>3 million patients treated globally3
Extremely well tolerated4
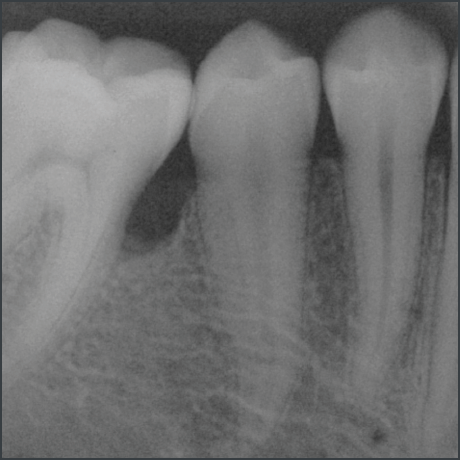
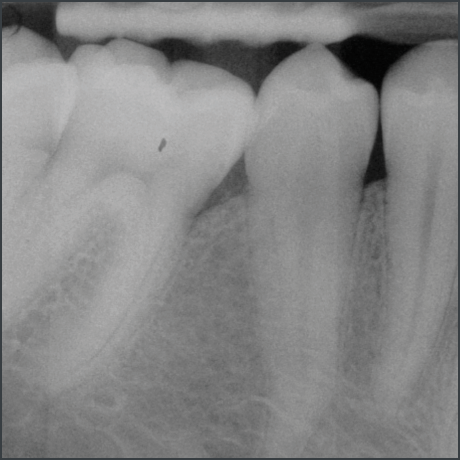
Stable results documented over 10 years in 2 indications5,10,11
Long term clinical case follow up10,11
Unique and pioneer6
Approved in +90 countries7
JOIN US AT

Wednesday, October 15, 2025 | Room 601 AB

Dr. Arndt Guentsch
Clinical Strategies with Emdogain: Turning Biology into Better Outcomes
1:00 p.m. – 1:45 p.m.

Dr. Shayan Barootchi
The Modern Landscape of Implant Therapy: Immediacy, Soft Tissue Engineering, and Digital Innovations
2:05 p.m. – 2:50 p.m.

Dr. Phil Walton
Guided Implant Workflows for Full Arch: Practically Speakings
3:10 p.m. – 3:55 p.m.

Dr. Luis H. Gonzaga
Singles to Full Arch: The Latest Innovations in Straumann’s Digital Workflow Through a Prosthetic Lens
4:15 p.m. – 5:00 p.m.
Features and benefits
Straumann® Emdogain® is backed by extensive clinical documentation. Over the past 30 years it has been used in over 3 million patients3 and documented in over 1,700 scientific publications.2
As it is a gel, Straumann® Emdogain® is easy to apply, even when the defect is difficult to access.
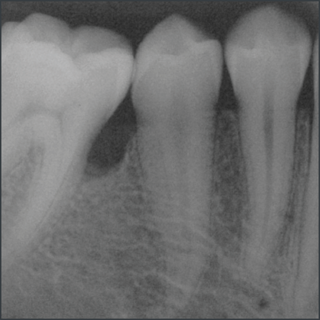
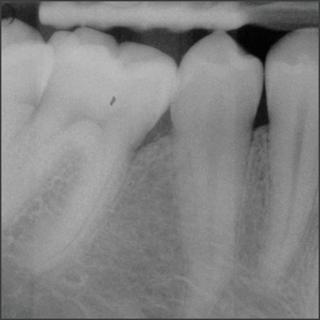
In a randomized clinical study Emdogain was compared to barrier membranes for treatment of mandibular buccal class II furcation defects. Both treatments led to significant clinical improvements. There was a significantly greater reduction in horizontal furcation depth with Emdogain. Patients treated with Emdogain also reported a lower incidence of postoperative pain and swelling at one week post op8.
A prospective multicentre randomized controlled clinical trial was executed to compare the papilla preservation flap surgery with or without Emdogain for treatment of intrabony defects. After one year, on average clinical attachment level gain and pocket reduction was significantly higher with Emdogain9.
WEBINARS

Dr. David Lipton
30 years with Emdogain®: History, Biology and Clinical Applications
Tuesday, September 9th | 7:00 PM EDT (New York)

Dr. Arndt Guentsch
Clinical Strategies with Emdogain: Turning Biology into Better Outcomes
Wednesday, November 19th | 7:00 PM EDT (New York)
SUBMIT A CASE
In recognition of 30 years of this advancing and groundbreaking solution, we invite clinicians to submit their case studies and be part of our 30 year anniversary casebook. Celebrate the progress in regenerative dentistry—and shape its future. Your work could inspire the next generation of clinical innovation!
To view the full terms and conditions for case submission requirements, click here.
All submissions must include the Straumann Case Evaluation Form and the provided template.
Straumann collects your information to facilitate the commercial relationship, contact you regarding future product offerings and promotion and all other permissible business purposes. Please see our Privacy Policy for more information.
Important Safety information:
Indications
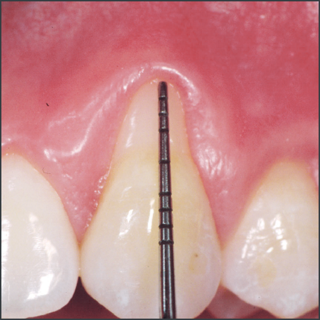
- Emdogain® has been shown to be effective in sites with periodontal pockets more than 6 mm associated with vertical bone loss on radiograph greater than 3mm.
- Emdogain® has also been shown to be effective with furcation involvements exceeding 2mm but not through-and-through defects.
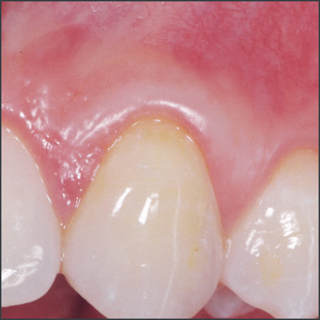
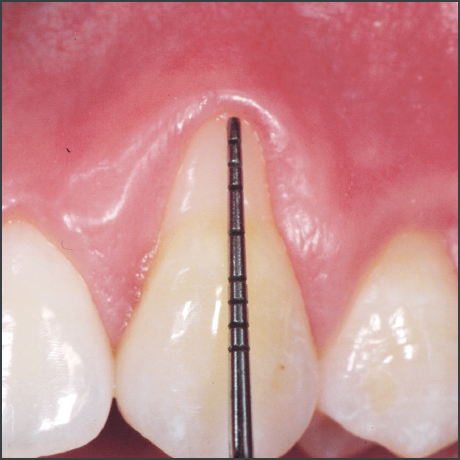
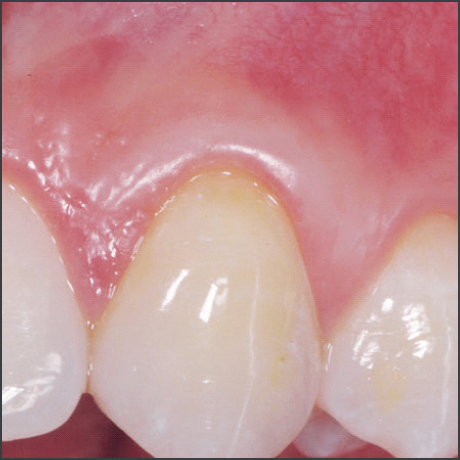
- Emdogain® used in recession defects has been shown to offer a potential for improved root coverage compared to the use of a coronally advanced flap alone, good aesthetic outcome, a gain in keratinized tissue and a potential for regeneration of attachment.
- When used with Bone Ceramic, Emdogain® is also indicated for use in extraction sites.
Contraindications
Based on the results of the risk analysis the following patient population are contraindicated: patients with disorders or conditions including, but not limited to the following: uncontrolled diabetes or other uncontrolled systemic diseases, disorders or treatments that compromise wound healing, chronic high dose steroid therapy, bone metabolic diseases, radiation or other immuno-oppressive therapy and infections or vascular impairment at the surgical site.
For complete labeling refer to ifu.straumann.com.