

bone & tissue regeneration
botiss is an innovative, clinically oriented medical and pharmaceutical company headquartered in Germany, with R&D and production sites in Germany, Austria, and Great Britain. Since the foundation of botiss biomaterials in 2009, botiss has become a leading developer, manufacturer and marketer of biomaterials for oral tissue regeneration.

Innovation and cooperation
With 50% of the 120-plus employees being scientists, botiss is constantly investing in research and further education. Unique innovations, the concept of high-quality learning and education with the botiss academy, and the international bone & tissue days are the results of our partnership with worldwide renowned academic research institutes, global opinion leaders, and practitioners in their daily clinical environment.
50% of botiss employees are scientists
Present in over 100 countries worldwide
more than
100 ongoing studies
The indication-oriented products are used worldwide in over 100 countries and are supported by both clinical studies and many years of clinical experience.
Our Vision
This is the driving force that leads us to a continuous development of new materials and to the optimization of established products.
Focus on the Clinician
The comprehensive range of clinically proven solutions of the highest quality includes membranes for guided tissue and bone regeneration, bovine, allogeneic and synthetic bone graft substitutes as well as products for soft tissue grafting. The botiss Regeneration System products have proven safety, efficacy and reliability in a variety of preclinical and clinical studies and daily clinical work with hundreds of thousands of patients worldwide.
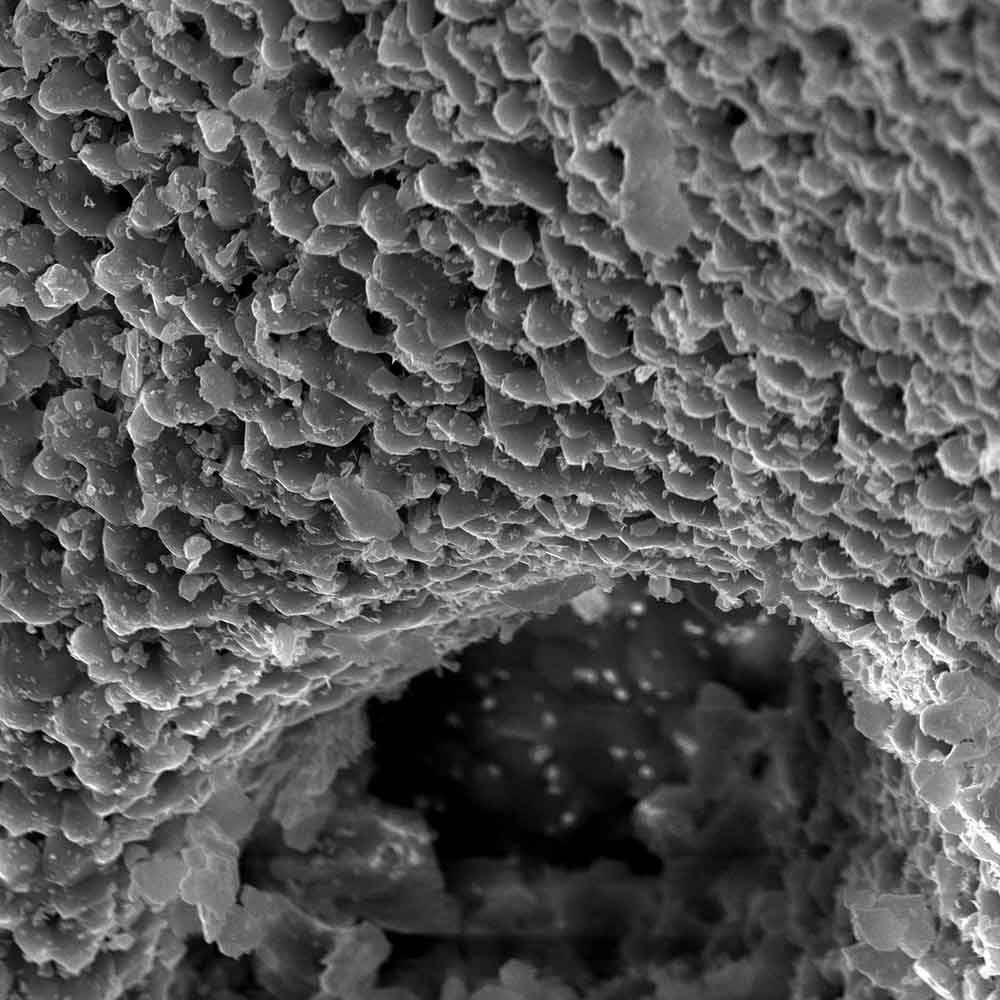
Patient safety, treatment comfort, reliable and predictable treatment results are your and our first priority.
Research
As a future-oriented company, botiss biomaterials invests substantially in cutting-edge research such as stem cells and growth factors.

Looking for new paths
Our worldwide cooperation with prominent research centers and key opinion leaders provides further impulse for pioneering clinical solutions and trends in the field of tissue regeneration.
Our innovative technologies are founded on longstanding academic and industrial research and testing.
Ongoing studies carried out in international research centers
Collaboration with research institutions around the world
Clinical and
scientific contributions

“Products from botiss are perfectly designed to match one another and to be used in combination for every single clinical situation, from sinus lift to horizontal/vertical augmentations or the improvement of the soft tissue quality.” Dr. Marius Steigmann
Development
We develop new, innovative and patient-tailored materials
and aim to optimize established indication-oriented products.
5+ million patients
were treated with botiss products worldwide
Manufacturing
The products of the botiss regeneration system have proven their success in terms of safety, efficacy, and reliability in a multitude of preclinical and clinical studies and, most importantly, in the daily clinical work with hundreds of thousands of patients treated worldwide.
All products are manufactured under the strictest quality controls, keeping our biomaterials at the highest scientific and quality standards. According to MDD 93/42/EEC botiss is a legal manufacturer of medical devices. Therefore, we have implemented a quality management system conforming to EN ISO 13485. botiss is pharmaceutical entrepreneur according to section 4 subsection 18 of the german medicines act (AMG).
