The introduction of Titanium alloys has illuminated new perspectives in contemporary implantology, enabling dental professionals to provide their patients with an expanded range of treatment possibilities. The ongoing research into Titanium alloys continues to uncover potential new applications and improvements. As our understanding of materials and the human body grows, the integration of interdisciplinary knowledge ensures that the next chapter in implantology will be even more promising and transformative.
Understanding osseointegration
Our comprehension of the processes governing osseointegration has experienced a remarkable evolution1. Initially, osseointegration was primarily interpreted as unimpeded bone formation around bioinert materials1–3. In the 1990s, cell and molecular biology research began focusing on surface-adherent osteogenic cells and later also osteoclasts, which allowed describing osseointegration and peri-implant bone apposition as the net sum of bone forming and resorbing processes4. Nowadays, researchers started describing osseointegration in a more comprehensive way5,6. The immune system, a previously frequently overlooked factor, is currently increasingly recognized to play a pivotal role in regulating and mediating the processes governing both short- and long-term integration of implants7,8. This novel osteoimmunological description of osseointegration has also revealed the importance of Macrophages9,10. Of significant importance was the finding that Macrophages, depending on their environment can shift their status from a secretory pro-inflammatory “M1” into a re-generative “M2” phenotype. The phenomenon of Macrophage polarization is now being considered a key determinant for the type, magnitude, and duration of the inflammatory response to an implanted material9–11. In conjunction with other processes, it determines whether an inflammatory response may resolve into a healing and osseointegration pattern or persist and trigger processes like fibrous encapsulation, bone resorption, and ultimately osseointegration failure12. Inflammatory processes can have a dual role in osseointegration. On the one hand, transient inflammatory reactions are vital in promoting bone formation and implant integration. Persistent inflammation is, however, closely connected to a bone resorptive pattern, which can ultimately negatively influence the long-term survival and success of osseointegrated implants12–14. Components of both the innate and adaptive immune systems have been found to influence peri-implant bone formation and loss. This finding supports the notion that the inflammatory status and response around an implant is dynamic, complex, and patient-specific8,13. The implant material and its putative migration into peri-implant tissues has recently received increasing attention as a possible nonplaque-related inflammatory co-stimulus for peri-implant bone loss8,13,15–18. While a unidirectional causative relation between this process and peri-implant bone loss remains controversial, the aspect itself supports the importance of implant materials13,19.
Do implant properties have an effect on immunomodulation?
Researchers have only recently discovered that the effect of implant features (the implant material itself, surface topography, and modifications) on its osseointegrative capacity is, to a considerable extent, based on immunomodulatory effects1. Specific implant surface modifications (e.g. hydrophilicity) have been shown to modulate the phenotypic response of adherent Macrophages simultaneously stimulating osteoblastic bone formation and inhibiting osteoblastic bone resorption12,14,20. Other studies have reported that modified Titanium and Titanium-Zirconium alloys were the most effective for inducing an anti-inflammatory phenotype in adherent macrophages, as indicated by significant changes in cytokine gene expression and secretion profiles10. The implant material, however, has long been considered a given in implant dentistry, whilst it may represent the most crucial variable governing osseointegration21. Based on their ideal material characteristics and mechanical properties resembling bone, Titanium has evolved into the material of choice for dental and orthopedic implants21. The onset of osteoimmunological aspects in implant research has strengthened the importance of the implant material on short- and long-term implant integration and revitalized the question if different implant material composition, (e.g. using Titanium alloys), may result in potentially osteoimmunologically superior properties when compared to pure Titanium12,22–24. Indeed, Titanium-Zirconium alloys have emerged as a valuable alternative to Titanium, providing access to implants with superior mechanical properties, and equivalent biomechanical behavior25–28. A potentially undervalued but equally important feature of Titanium Zirconium alloys is their excellent corrosion resistance, attributed to a more protective and resistant surface oxide layer compared to the one of Titanium24,29–31. The significance of this aspect is reflected by the potential to result in a more favorable immunological host-implant interaction32.
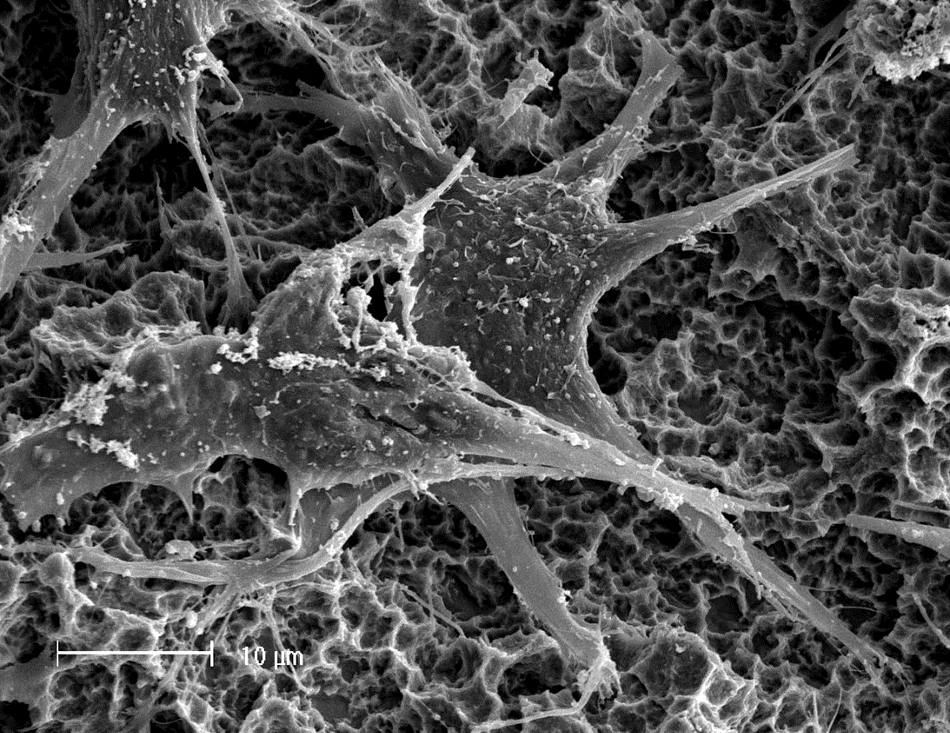
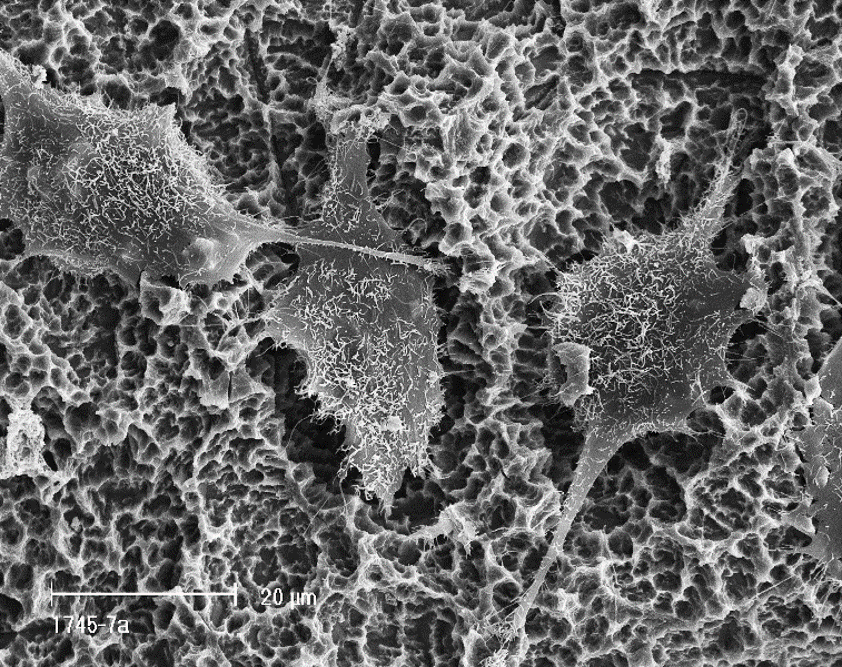
Straumann® Roxolid® - the Ti-15Zr alloy
Introduced in 2008, the Titanium-Zirconium alloy containing 13-15% of Zirconium, commercially recognized as Straumann® Roxolid®, and referred to further in this document as Ti-15Zr, has especially been appreciated for its superior mechanical strength. This feature has allowed for a reduction in implant dimensions, making treatments less invasive and more reliable in the long term. In an attempt to further elucidate potential osteoimmunological differences between Titanium and Ti-15Zr, researchers have compared the polarization state and cytokine release from macrophages in contact with corresponding implant surfaces. Results from these experiments indicated that macrophages in contact with Ti-15Zr, showed the greatest anti-inflammatory microenvironment and lowest pro-inflammatory factor release amongst the different implant materials tested20,33. Moreover, comparative in vivo examinations consistently reported differences in the healing kinetics around Titanium and Ti-15Zr implants, manifested by a more pronounced expression of a range of osteogenic factors and inflammatory cytokines26,34–38. Other research comparing the bone healing around Ti-15Zr and Titanium implants has suggested an impact of implant material on the bone quality around the implants, resulting in higher removal torque values measured for Ti-15Zr implants37, 39. These findings indicate that Ti-15Zr implants demonstrate a marked superiority over Titanium in both mechanical properties and biocompatibility. Therefore, the observed enhancement in long-term clinical performance of Ti-15Zr implants when compared to Titanium40 is neither surprising nor unexpected, and consequently aligns with the above-discussed research.
How may osteoimmunological benefits translate into benefits for clinicians and patients?
The notion of Ti-15Zr alloy being mechanically superior to Ti appears well established and complemented by scientific evidence. A more integrated perspective on osseointegration in the context of osteoimmunological considerations and on the properties governing associated short- and long-term implant outcomes indicates that the value of Ti-15Zr alloy not only be related to its improved mechanical properties but also to a potential biological and immunological advantage when compared to Titanium. It may also be acknowledged that implants with favorable osteoimmunological characteristics may be less contributory to an additive immunological response. Such implants may, therefore be considered for patients displaying immunologically-related risk factor profiles, including those with genetic deficiencies or under specific medication, as well as for patients and indications with increased risk of implant failure.
Dr. Marcin Maj holds the position of Head of Global Scientific Affairs at Institute Straumann in Basel, Switzerland
