Ceramic implants become increasingly popular and the scientific evidence confirming their clinical performance is continuously growing.
Read our updated scientific overview about this treatment alternative.
Introduction
Dental implants made of titanium and titanium alloys are considered a gold standard in implant dentistry due to their exceptional mechanical properties and long-term clinical success.1 However, the main drawback of a titanium implant is its dark gray color, which may occasionally be visible through the peri-implant mucosa, hence influencing esthetic outcomes, specifically in thin mucosal areas and anterior region. Therefore, a ceramic implant is turning out to be a more and more popular treatment option designed in anticipation of achieving a better esthetic outcome.
What is ceramic/zirconia?
The entry of zirconia transformed the market as a promising material with good mechanical properties, high biocompatibility and excellent esthetics, all of which encouraged researchers to investigate its possible use as a material for endosseous implants.2 The one used in the production of dental implants is yttria(Y2O3)-stabilized tetragonal zirconia polycrystal (Y-TZP) which has proven to be an attractive metal-free alternative to titanium as it exhibits more significant corrosion and wear resistance, an excellent flexural strength2-5 and furthermore, the compressive strength of zirconia implants is adequate in occlusion, and it can withstand occlusal loads for a more extended period.6

Fig 1:Straumann PURE implant
Straumann® has established an innovative manufacturing process followed by a rigorous 100% proof test in which every single implant from the Straumann® Implant System is tested mechanically before leaving the production site.
Is zirconia as good as titanium?
One of the most essential criteria for the success of implant treatment is osseointegration, which is the direct structural and functional interface between the living bone and surface of a load-bearing implant.7 Bone apposition takes place on different types of implant surfaces and greatly depends on the implant surface topography. The highest level of bone-to-implant contact (BIC) is associated with moderately rough surfaces.8 Therefore, it was crucial to obtain appropriate values of surface roughness on the zirconia implants. The surface of the Straumann® PURE Ceramic Implant System (ZLA®) is characterized by roughness values similar to those of Straumann implants with SLA® surface9 widely known from optimal surface topography that enhances BIC and facilitates osseointegration.10-15

Fig 2: The ZLA surface of the Straumann® PURE Ceramic Implant System
In preclinical studies, ceramic implants with ZLA® surface demonstrated similar healing and osseointegration as observed for the SLA® surface.16, 17 Also, removal torque values were equivalent to titanium SLA® implants.14 These reports were further confirmed by clinical investigations demonstrating survival rates of the monotype implants from 97.6% to 100% after one year. These are values within the range of reported one-year survival and success rates for a titanium or titanium alloy implants.18-21 A recent multi-center study reported survival and success rates of 97.2% after five years (manuscript in preparation).
Is it biocompatible?
The biocompatibility of an implant material depends on its chemical, physical, and structural properties that may influence the cell response at the tissue-material interface. Roughened Y-TZP was found to be an appropriate substrate for the proliferation and spreading of osteoblastic cells.22 Zirconia did not exert a cytotoxic effect on osteoblasts in vitro and made the cells capable of growth and development.23 When compared to titanium surfaces, the zirconia surface showed increased fibrinogen adsorption, platelet adhesion, activation, and thrombogenicity.24 Studies on bacterial adhesion on the zirconia surface determined that plaque formation on this surface might be less.25, 26 Also, a significantly reduced three-species biofilm thickness, human biofilm mass, and human plaque thickness was seen in vitro when compared to SLA surfaces.27 A higher degree of soft tissue integration around the ceramic implant than titanium was observed28 and an ideal papilla-crown proportion around zirconia implants was reported in a 3-year follow-up study.29
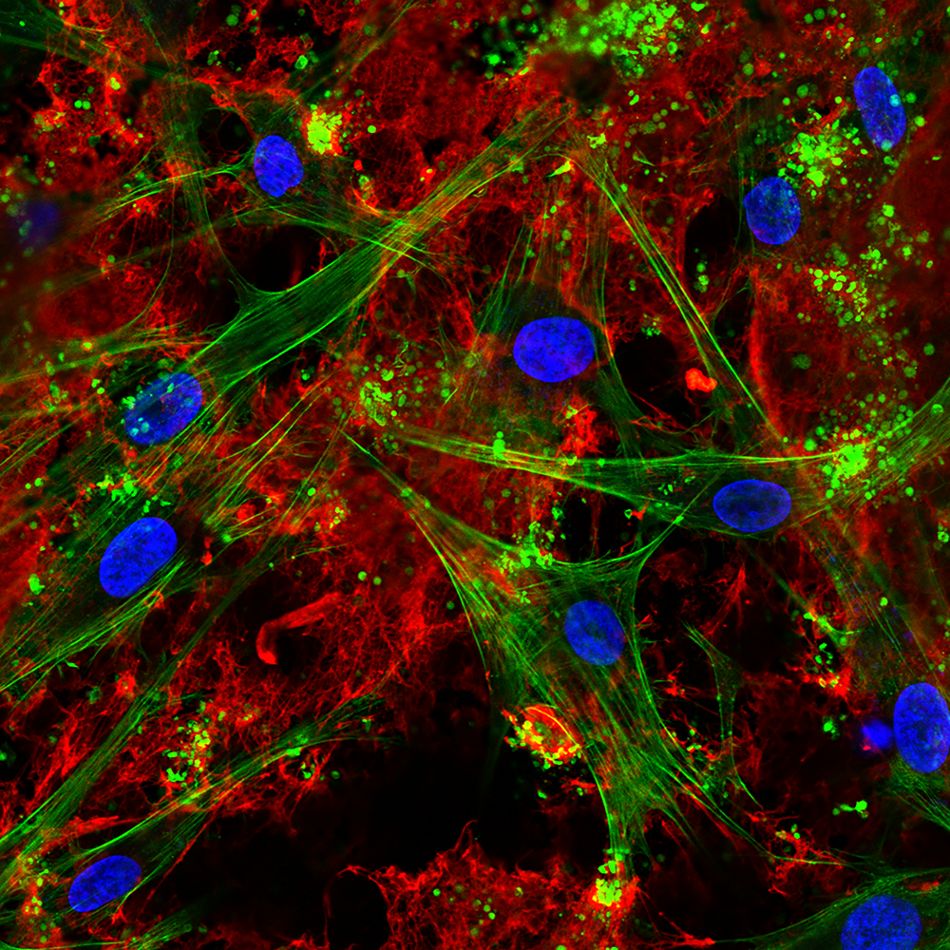
Fig 3: Confocal laser scanning microscopy visualizing seeded bone cells and pronounced fbrin network on the ZLA® surface after incubation in human whole blood. Fibrin network (red), actin cytoskele-ton (green), nuclei (blue). (Image courtesy: Dr. M. Rottmar)
What about the esthetics?
Most patients perceive treatment as successful when they are satisfied with the overall aesthetic appearance after the procedure. The Straumann PURE Ceramic Implant System is ivory-colored, which resembles natural tooth roots which is an advantage in patients with a thinner mucosal biotype or a high lip line smile.30-32
The review of peri-implant soft tissue color suggested that the color outcome might be influenced by both the implant and the abutment material. Ceramic components, when compared to metallic ones, appear to provide an improved color matching between peri-implant soft tissues and soft tissues around natural teeth. 33 Excellent esthetic outcomes and papilla formation around ceramic implants have been reported in several clinical studies,20, 21, 31 even for challenging indications.
Is it clinically proven?
Ceramic implants have rapidly demonstrated numerous benfits on par with titanium as an implant material in various clinical trials.12, 34, 35 They have had FDA approval since 2007, and the ceramic material has been used to make dental implants in Europe since 1987. As the technology and methods have evolved with titanium implants and undeniably the field of dentistry overall, many amendments have also been executed to the concept of ceramic implants that have significantly improved their standard.36 Ceramic implants become more and more popular treatment option, and the amount of published scientific evidence supporting their clinical application is continuously growing.12, 19, 34 There is more than enough data to confirm the long-term sustainability of zirconia dental implants, even by conventional standards.35
The Straumann® Ceramic Implant Systems are the result of more than 12 years of uncompromising research and development. They combine quality and precision, strength, clinical success, and flexible treatment protocols in an innovative solution that helps you to meet the needs of your patients. Moreover, the above discussed clinical evidence on excellent clinical performance confirms that ceramic implants can be considered a safe and predictable treatment alternative.
To know more about the implant line visit.