NAVIGATING PERI-IMPLANTITIS – ESSENTIAL FACTS AND FIGURES
Peri-implantitis refers to a bacterial plaque-associated inflammatory condition of the peri-implant tissues. Patients and clinicians may face various challenges based on the disease stage and progression, ranging from compromised esthetics to complete prosthetic loss and associated financial and psychological burdens1–3. Generally, peri-implant pathology can be classified into two fundamental stages: peri-implant mucositis and peri-implantitis2. While the former is reversible and affects only the peri-implant soft tissues, it may, if left untreated, progress into peri-implantitis. The latter condition is associated with a progressive loss of peri-implant crestal bone4,5. Therefore, the progressive and deteriorating nature of the disease underlines the importance of regular recall programs to detect the condition as early as possible6–8.
The reported prevalence of peri-implant pathologies shows significant variation. Individual reported values for peri-implant mucositis range from 19% to 65%, while those for peri-implantitis range from 1% to 47%9. Consolidated average values from systematic reviews of 43-47% and 20-22% for peri-implant mucositis and peri-implantitis, respectively, may serve as a good approximation for the occurrence of these conditions9,10. Next to cohort-related differences, the relatively high variation has been partly associated with inconsistent disease criteria and case definitions applied throughout different studies. This shortfall was only recently rectified as part of the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions held in 2017, where clear diagnostic and reporting classification criteria for peri-implant pathologies and their different stages were established2,11,12.
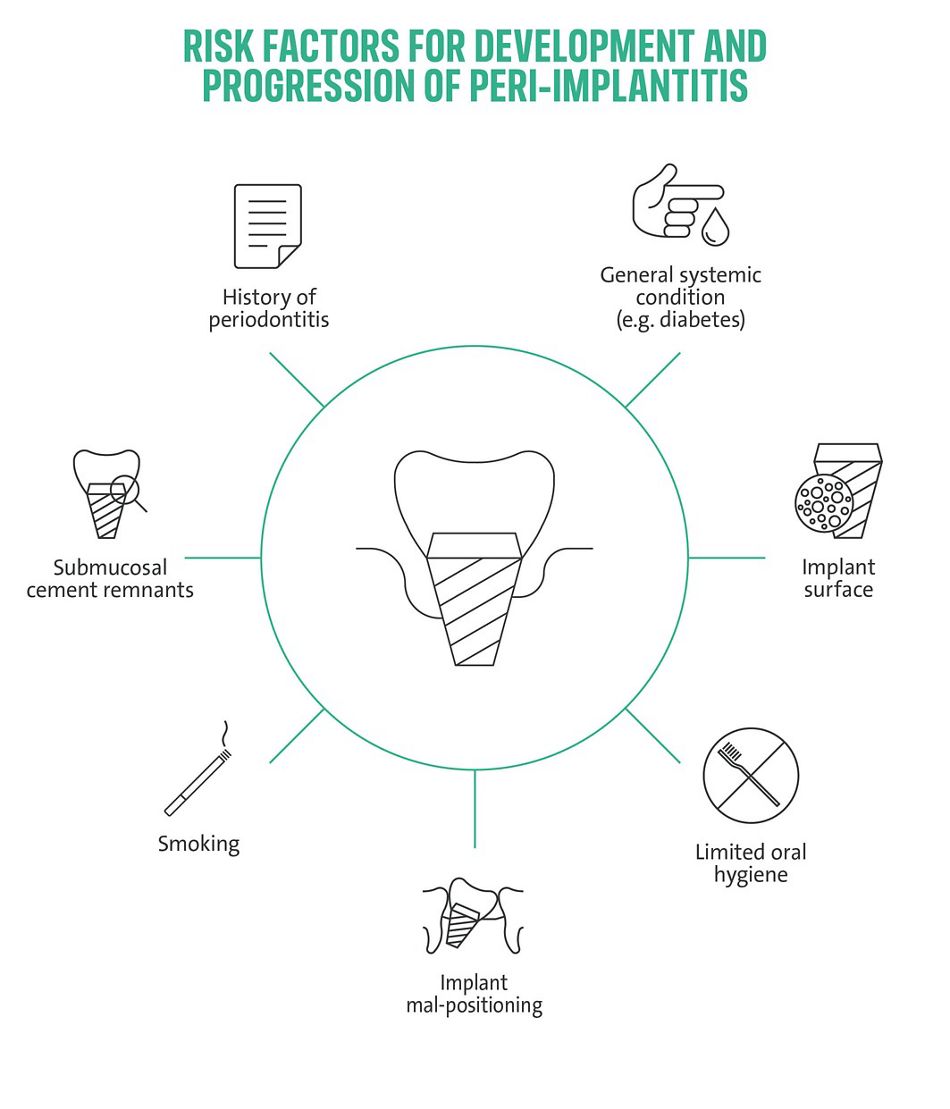
A vast and robust body of evidence supports the hypothesis that the primary etiology of peri-implant diseases is the accumulation of subgingival bacterial plaque1. Histological investigations have illustrated the detrimental effect of plaque-induced inflammatory reactions on peri-implant tissues. These studies indicated a limited capability of peri-implant tissues to prevent inflammatory cell infiltrates from progressing apically to underlying crestal bone structures compared to, e.g., periodontal tissues13,14. Despite this straightforward cause-effect relationship, the inflammatory response to subgingival plaque can vary significantly among patients12,15. These differences may at least partly be attributed to patient-individual risk profiles comprised of patient-behavioral, iatrogenic, local, and systemic factors and their aggregation14–17. Important risk factors/ indicators include a history of severe periodontitis, poor plaque control, and the failure to engage in regular supportive peri-implant care programs (SPIC)7,8,18. Further, an association between peri-implant disease and smoking, diabetes, and hyperglycemia appears commonly accepted, while attempts to prove this association by systematic reviews remain pending16– 19. Local factors include, e.g., the presence of submucosal cement, implant mal-positioning, or restorative designs limiting hygiene access16,18. There is a growing acknowledgment that patient-individual aggregated risk factor profiles are as crucial for peri-implantitis prevention and treatment as during implant placement itself17.
IS PERI-IMPLANTITIS RELATED TO THE TYPE OF IMPLANT SURFACE?
With subgingival plaque as the primary etiological factor for peri-implantitis, the question arises whether its onset and progression may be influenced by implant surface and material characteristics and their different propensity to promote plaque accumulation and maturation20,21. Most data addressing this aspect rely on preclinical models involving spontaneous progressing experimentally induced peri-implantitis14. Some evidence suggests that disease progression may be accelerated around implants with modified, i.e., moderately rough surfaces, compared to non-modified, i.e., turned surfaces23. In the preclinical realm, direct comparative studies have confirmed involvement of implant surface topography in the development and maintenance of peri-implantitis and influence of implant or surface types on the incidence of peri-implantitis26,27. A direct comparison of commercially available implants with different macro- and microtopography demonstrated a negative effect of certain implant surface properties on the development and treatment outcomes of experimental peri-implantitis with additive/anodized type of surfaces being significantly inferior to the subtractive and turned type of surface22,24,25. Moreover, epidemiological studies in larger patient cohorts indicated an association of moderate to severe peri-implantitis with specific implant surface types. Specifically, the analysis suggested a potential clinical superiority of implants functionalized with the long-term established sandblasted and acid-etched surface compared to anodically oxidized types28,29.
HOW CAN PERI-IMPLANTITIS BE TREATED?
The field of peri-implantitis treatment options is rapidly evolving30, and the selection of the treatment concept and prognosis of the treatment outcome differently depends on the disease stage and progression17,33,34. Treatment approaches can be classified into non-surgical and surgical therapies31,32. Non-surgical therapy targets primarily the causative infective plaque biofilm and represents a first-line intervention for peri-implant disease irrespective of disease progression17,31,32. It is pivotal in controlling inflammation, arresting disease progression, and reestablishing oral health, and ideally comprises a reassessment and control of risk factor profiles14,17. Non-surgical therapy can be effective in treating peri-implant mucositis and early-stage peri-implantitis6,17,30. At the same time, electrolytic cleaning remains unsuccessful for severe peri-implantitis or to influence the prognosis of surgical therapies17,31,35. On the other hand, surgical therapies become essential in treating moderate to advanced staged peri-implantitis17,33,34,36,37. They comprise access flap surgery, including resective approaches with apically repositioned flaps (APF) and guided bone regenerative (GBR) treatments36,37. The surgical therapies may also be combined with implantoplasty to prevent plaque reaccumulation on exposed endosseous implant surfaces30,38. Both non-surgical and surgical treatment approaches remain highly variable and non-standardized31. Therefore, the decisions for a specific treatment modality and its prognosis should always consider the treatment area, defect extension, and morphology17,30,34. As the prognosis for peri-implantitis treatment outcomes remains relatively variable, early-stage detection and treatment remain the hallmark in peri-implant disease management6,30,36,37,39. Implant surface decontamination can be considered pivotal to all non-surgical and surgical approaches30,39,40. Many mechanical, chemical, and physical methods have been proposed, often combined with local antiseptics or antibiotics17,31,32,41. Despite substantial efforts to further improve the efficacy of implant surface decontamination as a prerequisite for re-osseointegration, the validity and reliability of such techniques remain to be demonstrated31,40,41.
IS ELECTROLYTIC CLEANING DIFFERENT FROM THE EXISTING SURFACE DETOXIFICATION TECHNIQUES?
Implant surface access remains vital to all current mechanical cleaning methods, a parameter frequently constrained in peri-implantitis therapy for anatomical reasons or by the implant/restorative design itself41. Since bacterial biofilms develop a surrounding glycoprotein matrix that protects them against antimicrobials and antibiotics, disinfecting strategies require additional mechanical methods to disrupt the biofilm prior to disinfecting an implant surface42-43. One of them represents a recently emerged, novel treatment concept called electrolytic cleaning which allows for cleaning titanium implants affected by peri-implantitis44–46. It is based on the automated application of physiologically harmless low-voltage cathodic currents while flushing the coronal aspects of the implant with a buffered electrolyte solution47–50. The applied currents induce a well-controllable electrochemical reaction around the entire implant perimeter, independently from the level of access or the implant's micro or macro design49. In-situ formation of small amounts of hydrogen has been shown to lift and detach bacterial plaque50,51. The electrochemical reactions and the influence of electrolyte types were elucidated in detail, confirming the method's capability to detach bacterial biofilms from implant surfaces50. The use of electrolytic principles for the in situ disinfectant generation has been exploited in other application areas for some time52. However, the transfer of this principle to peri-implantitis treatments using standardized protocols and equipment was only achieved recently. The principle has been developed and standardized for peri-implantitis therapy under the name GalvoSurge.
EFFICACY AND CLINICAL EVIDENCE SUPPORTING THE ELECTROLYTIC CLEANING TREATMENT CONCEPT
The efficacy of the electrolytic cleaning system was addressed in various pre- and clinical studies. First, in-vitro studies demonstrated the decontaminating effect of electrolytic cleaning on multispecies biofilms compared to other commonly applied implant-surface cleaning methods, including powder-spraying, diode lasers, and plasma cleaning49,51. These studies also demonstrated that electrolytic cleaning could eliminate or neutralize surface-adherent bacteria, yielding nearly sterile implants, unable to induce bacterial growth when immersed in a cell growth medium51. Another preclinical study involving ligature-induced peri-implantitis histologically compared the healing of electrolytically cleaned to conventional, H2O2-based, ablatively cleaned, and augmented peri-implantitis sites. This study indicated that electrolytic cleaning, compared to corresponding controls, may provide the prospect for complete re-osseointegration, an observation previously considered hardly achievable40,47. Promising, positive outcomes were demonstrated as well as part of clinical trials. These trials confirmed the absence of inflammation and complete or significant bone fill, depending on defect morphology in electrolytic cleaning-treated sites. Moreover, no additional mechanical cleaning was needed or beneficial after the cleaning procedure48. Human histological investigations from follow-up time points of the same cohort followed by independent studies on explanted implants lost due to treatment – independent complications confirmed the prospect of electrolytic cleaning to result in implant surfaces conducive to complete re-osseointegration53,54.
The growing body of evidence confirms the importance of early detection, risk factor control, and the implementation of tailored treatment strategies based on the disease stage and progression. The recent advances in understanding and managing peri-implantitis represent a significant step forward in implant dentistry. Moreover, the continuous refinement of diagnostic criteria and classifications and the development of innovative treatment approaches offer renewed hope for effective management and potentially complete resolution of peri-implantitis. As research continues to evolve, these advancements will help to enhance clinical outcomes, patient satisfaction, and the long-term success of implant therapy.
Dr. Marcin Maj holds the position of Head of Global Scientific Affairs at Institute Straumann in Basel, Switzerland
