Introduction
Peri-implantitis is a biological complication that affects a growing number of patients with dental implants. Characterized by inflammatory changes in the peri-implant soft tissues and progressive loss of supporting bone, it poses a significant threat to implant survival if not appropriately managed. Effective treatment remains a clinical challenge, particularly due to the difficulty in achieving thorough decontamination of the implant surface and promoting predictable re-osseointegration.
This case report presents the surgical and regenerative management of peri-implantitis in a 56-year-old female patient with a previously restored implant in the posterior maxilla (region #16). The patient presented clinical signs of inflammation and radiographic evidence of vertical bone loss, despite adherence to supportive periodontal care and absence of systemic risk factors. A comprehensive treatment plan was developed, combining non-surgical therapy, surgical debridement, and guided bone regeneration (GBR), with the incorporation of electrolytic decontamination using the GalvoSurge® solution.
Electrolytic cleaning offers a novel approach for implant surface decontamination, aiming to disrupt the biofilm at a microscopic level without mechanically altering the implant surface. Its use in this case was integrated into a regenerative protocol involving autologous bone, xenograft material, and a non-resorbable membrane. The aim was to restore peri-implant health and preserve the existing prosthetic restoration through a minimally invasive, biologically driven approach.
Initial situation
A 56-year-old female patient presented with the chief complaint of recurrent inflammation in the mucosa of region #16, accompanied by occasional bleeding and exudate. Her primary expectation was to resolve the inflammatory process and recover the health of the tissues surrounding the implant. The patient reported no systemic risk factors. Her medical and dental history included the extraction of tooth #16 in 2014, followed by implant placement in the same region that year and prosthodontic restoration in 2015. The patient has since received regular supportive implant therapy. There was no history of periodontitis or other significant dental disease.
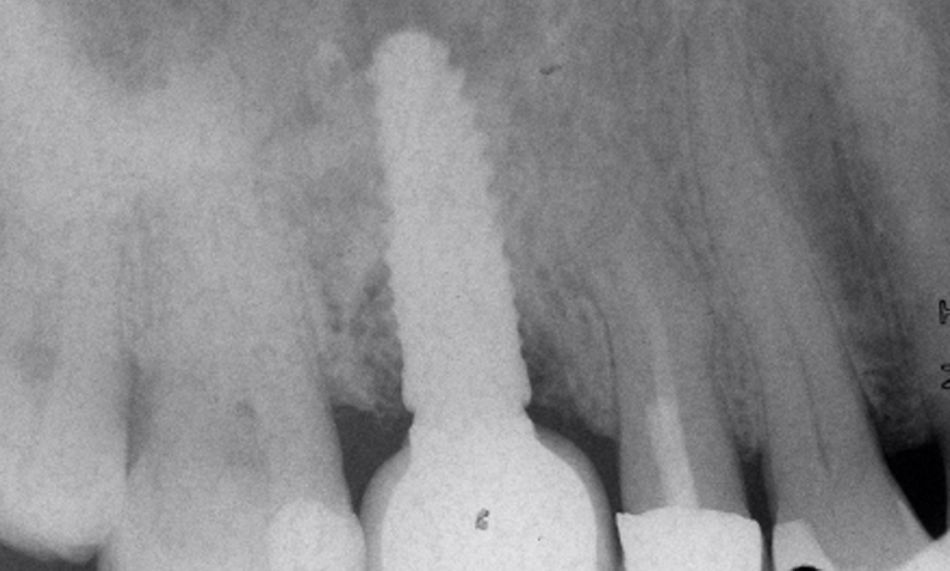
The intraoral examination showed localized inflammation around the implant in region #16, characterized by redness, swelling, bleeding on probing, and the presence of suppuration. The radiographic evaluation revealed vertical bone loss adjacent to the implant, while horizontal bone availability and bone quality remained within acceptable limits. The prognosis of the remaining dentition was favorable based on the clinical and radiographic findings (Fig. 1).
Comprehensive clinical and radiographic findings led to the diagnosis of peri-implantitis at region #16.
Treatment planning
The treatment workflow included:
- Non-surgical periodontal therapy: Professional tooth cleaning and oral health instructions. Local antibiotic treatment based on doxycycline 14 days before surgery, in combination with subgingival debridement using Labrida BioClean®, whose fibers are made of chitosan, a material with documented bacteriostatic and anti-inflammatory properties (Figs. 2–8).
2. Surgical treatment: GalvoSurge®, GBR using autologous bone, Straumann® XenoGraft, and permamem® membrane.
3. Second-stage surgery: prosthetic rehabilitation refixed on implant #16.
4. Follow-up visits for control.
Surgical procedure
The surgical procedure was planned under local anesthesia combined with intravenous sedation using midazolam to ensure anxiety control and patient comfort. A full-thickness flap was raised for the augmentation and to allow access to the peri-implant defect, followed by meticulous degranulation and mechanical implant surface decontamination (Figs. 9–13).
Following prosthetic screw removal, the GalvoSurge® system was applied for implant surface decontamination (Figs. 14–16).
Local autologous bone was harvested using a Safescraper® and combined with a xenograft (Straumann® XenoGraft) to perform guided bone regeneration (Figs. 17–19).
A synthetic PTFE membrane (Permamem® membrane) was used to stabilize the graft (Figs. 20 – 22). A flap advancement was performed and closed primarily without tension (Fig. 23).
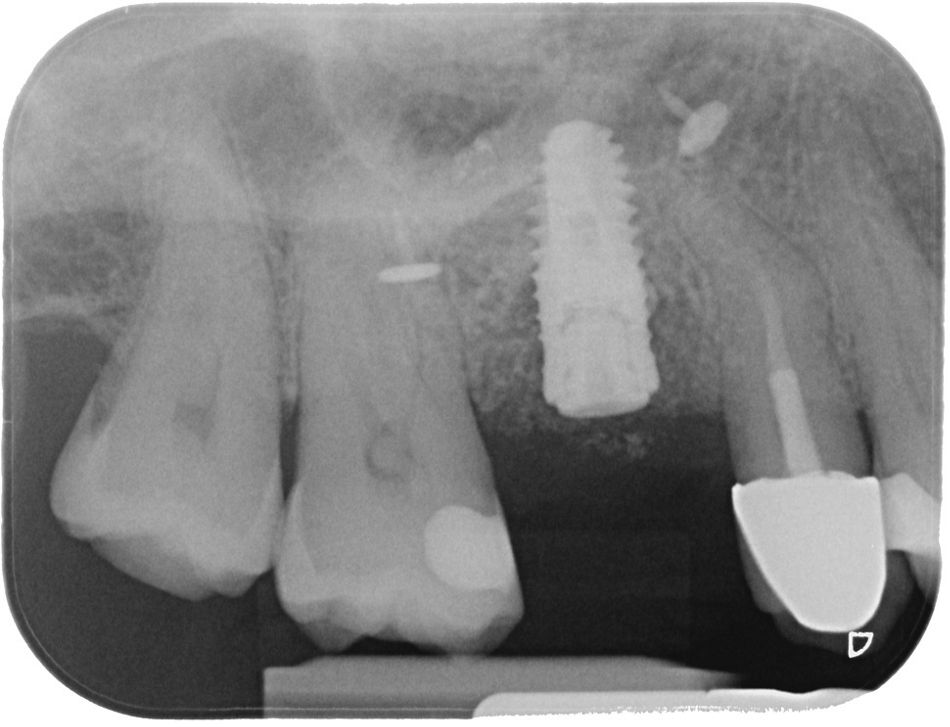
Upon completion of the first-stage surgery, a control radiograph was taken, revealing the membrane fixation pins and confirming that everything was in good condition (Fig. 24).
Four months after the surgical procedure, a second procedure was performed to remove the screws and improve the soft tissue contour. A partial-thickness flap was raised for soft tissue management. After reflection of the periosteum, the pins and membrane were extracted, and the previously removed prosthodontic restoration was reinserted (Figs. 25–28).
A small graft of palatal tissue was then harvested to improve the soft tissue conditions around the implant, and the flap was sutured (Figs. 29,30).
Postoperative care included appropriate antibiotics, analgesics, and chlorhexidine mouth rinses.

A Center of Dental Education (CoDE) is part of a group of independent dental centers all over the world that offer excellence in oral healthcare by providing the most advanced treatment procedures based on the best available literature and the latest technology. CoDEs are where science meets practice in a real-world clinical environment.
Prosthetic procedure
The original screw-retained prosthesis was reattached. The patient was enrolled in a 12-month follow-up program to monitor wound healing, tissue stability, and prosthetic function (Figs. 31–33).
Treatment outcomes
The treatment of peri-implantitis remains a clinical challenge due to its complex etiology. In this case, a combined non-surgical and surgical approach was used to achieve reosseointegration and soft tissue stability. Initial non-surgical therapy included subgingival debridement with Labrida BioClean®, a chitosan-based device known for its bacteriostatic and anti-inflammatory properties. These have shown promising results in improving outcomes in peri-implant therapy due to their biocompatibility and antimicrobial action5, along with local doxycycline to reduce inflammation and improve tissue handling during surgery. The combination of decontamination using GalvoSurge® and biological regeneration reflects established strategies that highlight the critical role of surface decontamination prior to grafting procedures to achieve successful reosseointegration.6,7 An in vitro study demonstrated that the electrolytic cleaning was significantly more effective at inactivated bacterial biofilms compared to a powder-spray system.8
Subsequently, regenerative surgery in combination with GalvoSurge® decontamination, autologous bone, xenograft, and a non-resorbable membrane was performed. At 4 months, a second intervention optimized soft tissue contours using a partial-thickness flap and palatal grafting. Moreover, soft tissue refinement through palatal grafting has been identified as a key factor in achieving long-term stability and esthetic integration.9
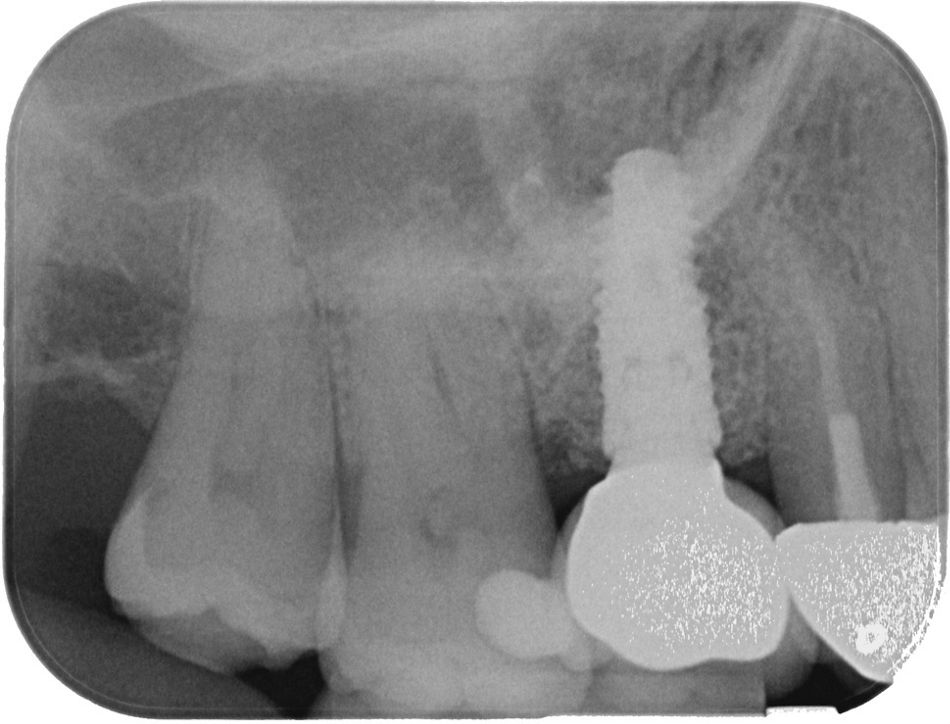
At the 12-month follow-up, the implant in region #16 showed healthy peri-implant tissues, with no signs of inflammation, bleeding on probing, or suppuration. Probing depths remained within normal limits, and radiographic evaluation confirmed stable marginal bone levels. The reinserted prosthesis worked without complications, and occlusal parameters remained stable. Both the patient and the clinician were highly satisfied with the outcome, particularly due to the resolution of symptoms, the preservation of the original prosthesis, and the regeneration of lost bone.
This case highlights the effectiveness of the potential for combining electrolytic decontamination with GBR to achieve favorable functional and biological outcomes in the treatment of peri-implantitis, even in complex scenarios.
Author’s testimonial
The combination of GalvoSurge® with GBR and a non-resorbable membrane greatly improved my defect fill results. In my clinical practice, complete re-osseointegration is no longer unpredictable.
The patient stated, “I am happy that all my inflammatory problems have been completely resolved. I was afraid of losing the implant after I saw the defect on the X-ray. It is amazing that it could be completely regenerated.”