A recent systematic review reported a 22% prevalence of peri-implantitis2. However, different percentages of prevalence have been reported due to the various definitions of this pathological condition. Therefore, depending on the study analyzed, it can vary from 1% to 47%3. Moreover, it has been suggested that the described bone loss is time-dependent and that the follow-up time of the different studies can also affect the percentage of prevalence described4, 5.
The treatment of peri-implantitis aims to resolve the inflammation of the soft tissues and stop the subsequent peri-implant bone loss. Recent systematic reviews have reported that regardless of the non-surgical treatment modality used, it is insufficient to stop the disease. In contrast, surgical treatment has shown greater efficacy and in the longer term7,8. Furthermore, it has been demonstrated that factors such as the implant's surface significantly influence the results of surgical treatment8,9. In addition, the anatomical configuration of the peri-implant bone defect has been shown to be another relevant factor, especially when selecting the type of surgical approach to be performed10. The goal of reconstructive procedures for peri-implant bone defects is to restore the implant support tissues11,12 and thus improve aesthetics and achieve a hypothetical re-osseointegration13.
The potential benefit of using bone substitutes/biological agents in reconstructive procedures for the treatment of peri-implantitis remains undefined due to the limited number of clinical studies with very heterogeneous designs and different follow-up times.
Concerning the material that should be used during the reconstructive procedure, the existing literature is also heterogeneous. Several studies have evaluated the effectiveness of material without comparing it with any control group, while others either compare the use of a material with the performance of only mechanical debridement or with the use of a different one14,15,16. For this reason, it isn't easy to draw solid conclusions about the ideal material.
A case series in which a xenograft was proposed to reconstruct peri-implant bone defects obtained predictable results in PPD and radiographic bone filling17. In addition, they reported no change in the level of the peri-implant mucosa during the entire follow-up.
Another case series proposed using the xenograft with a resorbable collagen membrane within a combination of regenerative-resective therapy of the intraosseous defect and the supra-osseous portion18. It was reported satisfactory results both in radiographic bone filling and reduction of PPD and the attachment level at 12 months of follow-up. In this case, there was a 1.3 mm increase in mucosal recession compared to the baseline situation. Recent investigations with even seven years of follow-up highlighted the importance of implant surface characteristics in this type of therapeutic approach9, 19.
If the results of the two described case series are analyzed, it could be concluded that the additional use of a membrane is contradictory. However, clinical trials comparing the additional use of a membrane in combination or not with the xenograft are necessary to obtain a conclusion. In this line, different studies with follow-ups of up to 5 years have described no statistically significant differences in favor of the additional use of a membrane and a high rate of complications related to the membrane exposure in the test group (44% after 2 weeks of healing)20,21,22.
Another research group that analyzed the additional benefit of using a resorbable membrane in combination with the xenograft did not report statistically significant differences in the variables at 6 months of follow-up23, while it showed better results in favor of the additional use of a membrane at 2 and 3 years of follow-up24,25.
In this way, it could be said that the additional use of a membrane is currently a controversial topic and that randomized clinical trials are needed to understand the benefit that could be achieved, taking into account the exposure risk.
The objective of the present clinical case is to describe the surgical approach of a reconstructive therapy to treat an intrabony peri-implant defect, using a collagenated xenograft after a peri-implant surface debridement with a chitosan brush.
Initial situation
A 35-year-old male presented to a periodontal maintenance visit in 2020. The patient presented bleeding on probing and deep peri-implant pockets around implant #25. In addition, in the periapical x-ray, an osseous defect with an intrabony component was detected in the distal aspect of the implant (Fig. 1).

Want to stay up to date?
youTooth.com is THE PLACE TO BE IN DENTISTRY – subscribe now and receive our monthly newsletter on top hot topics from the world of modern dentistry.
Presurgical phase
The patient received a full-mouth ultrasonic debridement and a manual curettage 4 weeks before the surgical protocol was performed. As a result, the patient reached full-mouth plaque and full-mouth bleeding scores below 20% before proceeding with the surgical treatment.
Surgical phase
The surgical procedure was performed under local anesthesia (Lidocaine HCL 2% with 1:100,000 epinephrine), and the following surgical intervention was carried out after removing the implant-supported prosthesis:
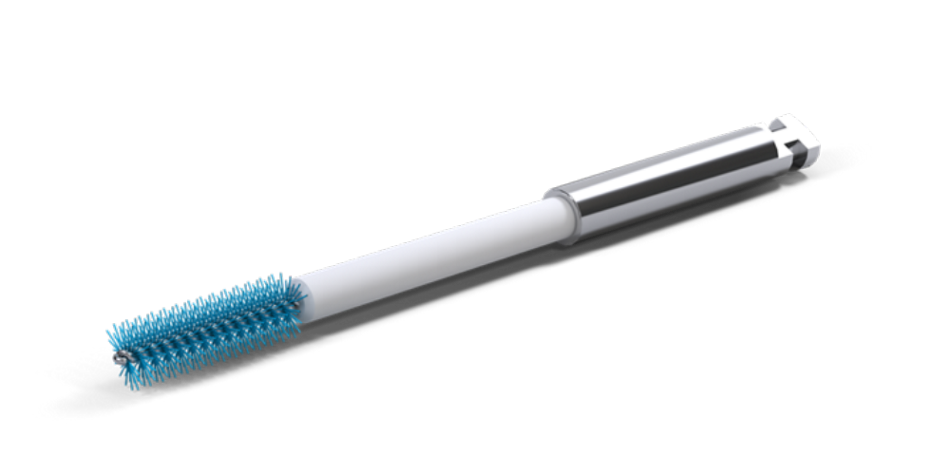
An intra-sulcular incision at the affected implant was made using a 15C blade. The flap was extended mesially, with a vertical releasing beveled incision making one tooth away from the affected implant. After full-thickness flap elevation and removal of granulation tissue, the exposed implant threads were debrided using a chitosan brush (Labrida BioClean™, Straumann, Basel, Switzerland).
The intraosseous component was grafted with a collagenated xenograft (Straumann® XenoFlex, Institut Straumann AG, Basel, Switzerland). The graft was adapted to the defect morphology. Primary wound closure was achieved using internal vertical mattress sutures in mesial and distal, and the gingival recession of #24 was covered, advancing the flap coronally. (Fig. 2-7)
Post-operative care
The patient was instructed to apply chlorhexidine gel in the area three times a day for 2 weeks. Furthermore, systemic amoxicillin 500mg (3 tablets a day for 7 days) and anti-inflammatory medication (Enantyum 25mg, 1 tablet every 6-8 hours for 4 days) were also prescribed.
Treatment outcome
After 2 weeks, primary wound closure was observed, and therefore, sutures were removed, and the implant-supported crown was repositioned. (Fig. 8-9)
Moreover, at 6 months follow-up, the radiological and clinical examination indicated a successful bone regeneration around the implant. (Fig. 10-11)
Same situation is observed at 12 months follow-up. (Fig. 12-13)
Conclusions
- The proposed implant surface decontamination with a chitosan brush seems to be safe and efficient.
- The proposed surgical reconstructive approach using a collagenated xenograft may offer a successful therapeutic option to reconstruct intrabony peri-implant defects (Fig. 14).
Do you want to find more about this topic? Then access the on-demand Biomaterials Online Symposium here: