What is this about?
- Learn how to manage peri-implantitis in a previously successful implant case, presenting with bleeding, suppuration, deep pockets, and radiographic bone loss.
- Step-by-step treatment approach including prosthesis removal, non-surgical procedures, electrolytic implant decontamination with GalvoSurge®, and GBR with particulate bone and a non-resorbable membrane.
- Clinical and radiographic outcomes after 4 months, demonstrating bone regeneration, implant stability, and restoration of function and esthetics.
Introduction
Peri-implantitis poses one of the most significant clinical challenges in modern implant dentistry, and is characterized by inflammation, bleeding on probing, and progressive bone loss around osseointegrated implants. While the etiopathogenesis is multifactorial, bacterial biofilm on the implant surface remains the principal trigger, undermining long-term implant success and requiring a comprehensive therapeutic approach.
One of the most critical and historically difficult aspects of managing peri-implantitis is the effective decontamination of the implant surface. Conventional mechanical or chemical methods may fall short in eliminating entrenched biofilms, especially within the micro-roughened surfaces of implants. Inadequate decontamination often leads to compromised outcomes in reconstructive procedures such as guided bone regeneration (GBR).
To address these limitations, electrolytic decontamination has emerged as a state-of-the-art technique, offering a minimally invasive yet highly effective approach to biofilm removal. GalvoSurge®, an electrolytic cleaning system, represents this modern modality, enabling removal of bacterial biofilms while preserving the microstructure and integrity of the implant surface.
The GalvoSurge® system works by applying an electrolyte solution to the exposed implant, followed by the application of a low-voltage electrical current. This generates hydrogen gas bubbles at the implant surface, which mechanically dislodge the biofilm without causing mechanical abrasion or damaging the implant surface. The result is a biologically clean surface that is suitable for re-osseointegration and optimal regenerative outcomes.
The following case report illustrates the importance of implant decontamination using GalvoSurge® in an 82-year-old patient who developed peri-implantitis 4 years after successful implant placement and prosthetic rehabilitation in the lower right quadrant. The affected implant (site #46) showed classic signs of peri-implantitis such as bleeding, suppuration, deep probing pocket depth and radiographic bone loss. After the removal of the prosthetic restoration and non-surgical procedures, the electrolytic cleaning with GalvoSurge® was performed, followed by a GBR procedure using particulate bone and a non-resorbable membrane.
Four months postoperatively, re-entry confirmed successful bone regeneration and implant stability, enabling reinstallation of the prosthetic bridge with restored function and esthetics. Radiographic follow-up demonstrated re-established crestal bone levels and absence of inflammation, affirming the benefit of thorough decontamination and guided regeneration.
Initial situation
In 2016, an 82-year-old female patient visited our practice with the main concern of difficulty chewing. She expressed a strong wish to restore posterior function in the lower right jaw with dental implants. Her primary goal was to regain functional ability, with long-term oral health as her secondary priority.
The patient was in good general health, with no significant systemic conditions, a non-smoker, and not taking any medication at the time. At the time of implant planning, there was minimal plaque and localized gingival inflammation. Radiographic evaluation revealed horizontal bone loss in the 4th quadrant, requiring bone augmentation.
Three implants were placed in the 4th quadrant, in sites #47, #46, and #44, along with bone regeneration (sausage technique). This procedure was performed to increase the available bone for implant placement using bone grafts, resorbable membranes, and fixation with screws to stabilize the graft and protect the regeneration site. A bridge was subsequently placed over the implants in positions #47, #46, and #44 (Figs. 1-3).
The patient returned in 2020 due to discomfort and bleeding associated with one of the implants. Clinical examination revealed inflammation, bleeding on probing, and suppuration around the implant in site #46. Radiographic evaluation showed both horizontal and vertical bone loss surrounding the implant, indicative of peri-implantitis and progressive bone resorption (Figs. 4,5).
Treatment planning
A two-phase surgical protocol was planned for the management of peri-implantitis at implant site #46.
Phase 1: Initial surgical and regenerative therapy
- Removal of existing prosthetic crown to allow full access to the implant surface and peri-implant tissues.
- Implant surface decontamination with GalvoSurge® electrolytic cleaning system to eliminate biofilm while preserving titanium integrity.
- Adjunctive mechanical debridement to remove residual granulation tissue.
- Guided bone regeneration (GBR):
- Particulate autogenous bone graft harvested locally.
- Placement of a d-PTFE membrane, stabilized with titanium pins.
- Advancement of buccal and lingual flaps to achieve primary closure.
- Postoperative management:
- Systemic antibiotics.
- Chlorhexidine mouth rinses.
- Detailed postoperative care instructions.
- Clinical and radiographic follow-up to monitor healing.
Phase 2: Re-entry and prosthetic rehabilitation (after 4 months)
- Re-entry surgery performed to:
- remove the d-PTFE membrane.
- assess bone regeneration and soft tissue healing.
- Confirmation of successful healing and bone regeneration.
- Surgical site closed after membrane removal.
- Reinstallation of prosthetic crown to restore function and occlusion.
Author’s testimonial
The procedure progressed smoothly, with good bone regeneration and confirmed osseointegration at re-entry. Soft tissues healed well, and radiographs showed stable bone levels. The use of GalvoSurge® was key in allowing us to preserve the existing bridge, avoiding its replacement and simplifying the restorative phase.
Surgical procedure
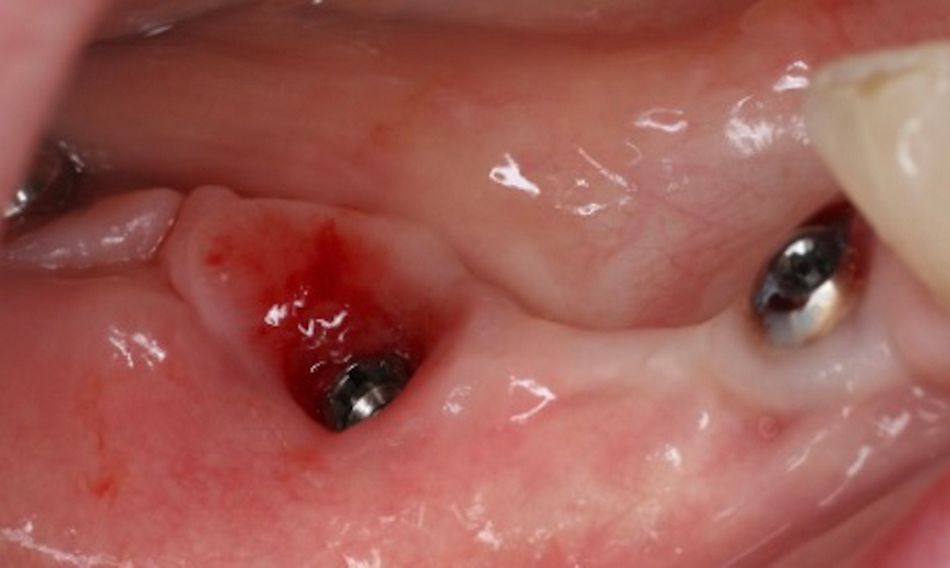
Local anesthesia was administered, and the implant-supported bridge was carefully removed to allow access to the affected area (Fig. 6).
The implant surface at site #46 was decontaminated using the GalvoSurge® electrolytic cleaning system, following the manufacturer’s protocol. The procedure involved the application of an electrolyte solution directly to the implant surface, followed by the activation of a low electrical current. This process generated hydrogen bubbles, which effectively detached the biofilm from the implant surface without mechanical abrasion. The area was then thoroughly irrigated with sterile saline to remove any residual debris (Figs. 7,8)
Following decontamination, the site was prepared for the guided bone regeneration procedure. The GBR was carried out using particulate bone graft material and a d-PTFE membrane, which was carefully secured with titanium fixation pins to ensure stability and proper space maintenance. The surgical site was sutured to achieve primary closure (Figs. 9,10).
The patient was then prescribed systemic antibiotics and chlorhexidine mouth rinses, along with detailed postoperative instructions, including a soft diet. Healing was closely monitored through clinical evaluations and radiographic follow-up.
After 4 months of uneventful healing the site was re-entered to remove the membrane. A complete bone regeneration was achieved. The implant appeared re-osseointegrated with the regenerated bone (Figs. 11-14).
Healing abutments were placed, and the site was closed again.
Prosthetic procedure
After a few weeks, an autogenous strip gingival graft was performed to increase the keratinized tissue (KT) on the buccal side, and the prosthetic bridge was reinserted. Note the adequate KT on both buccal and lingual sides (Figs. 15-18).
Control follow-up radiographs were taken at 1 year and 4 years, showing favorable conditions and confirming stable crestal bone around the implant. Note that the implant seems to be re-osseointegrated (Figs. 19,20).
Treatment outcomes
The site showed good bone regeneration and stable osseointegration at the time of re-entry. Soft tissue healing was uneventful, and radiographs confirmed stable bone levels. The use of GalvoSurge® allowed for effective decontamination, making it possible to preserve the existing bridge from 47–46 to 44.
Main learnings from this case
- Electrolytic decontamination with GalvoSurge® combined with GBR can successfully manage peri-implantitis, restoring both bone support and implant stability.
- Clinical and radiographic evaluation confirmed regeneration of crestal bone levels, resolution of inflammation, and re-establishment of peri-implant health.
- The case demonstrates that thorough implant surface cleaning is a key factor for predictable outcomes and long-term functional and esthetic success.