These implants have had an SLA® surface since 1997 and a SynOcta® abutment connection since 1999. They basically correspond to the Straumann® Tissue Level Implant System available today. Since 2005 the implants have been optionally provided with an SLActive® surface, which is scientifically proven to accelerate osseointegration even in compromised patients. Since 2009 all implants have been made of the extra strong titanium-zirconium alloy Roxolid®.

The Straumann® Tissue Level Implant Line
This iconic implant was designed for one-stage surgery to simplify handling and reduce treatment complexity
Basic research for the first Tissue Level generation was done by a group led by the Swiss dentist Prof. Dr. André Schroeder (University of Bern, Switzerland). Along with Per-Ingvar Brånemark, Schroeder was one of the fathers of modern oral osseointegration2. One of Schroeder’s scholars at the time, Prof. Dr. Daniel Buser, was a co-developer and the first clinical user of the ITI Bonefit® system, which led to the modern Tissue Level concept.
Clinical concept
From the very beginning two-part ITI Bonefit® and today’s Straumann® Tissue Level implants were designed for a one-stage surgical protocol. This involves transgingival healing, which eliminates the need for a second-stage surgery to expose the implant shoulder. In comparison with bone level implants, impression-taking and the handling of healing caps, abutments and prosthetic superstructures are significantly more straightforward, making daily clinical practice significantly easier. The protocol also saves time and reduces the complexity of the overall treatment.
Tissue stability – minimally invasive
The Straumann® TLX Implant system was designed for tissue stability and minimally invasive surgery – including non-augmentation cases.
Learn more about the potentials of the Straumann® TLX Implant System:
Another important aspect of the original Straumann® Tissue Level Implant System is its biomimetic design. The Standard type implant has a machined transgingival part continuous with the endosteal screw. The vertical dimension of this section gives a height of 2.8 mm (Standard implants), which is in the range of the mean biologic width of natural teeth3, 4. Thanks to this design the inevitable microgap between the implant shoulder and the abutment or restoration can be positioned at the gingival margin – away from the bone. Moreover, the smooth surface of the transgingival collar facilitates hygienic maintenance, which helps to reduce the risk of peri-implant infection and disease (see next section)5.
According to Professor Buser, after bony remodeling during the osseointegration process the micro-rough (SLA®/SLActive®) part of the implant should be completely covered with healthy bone. This applies to placement both into native or augmented bone and is an important prerequisite for long-term peri-implant tissue stability.
Scientific documentation
With more than 35 years of clinical application, Straumann® implants placed according to the Tissue Level concept are among the best documented in the market. Over a period of 10 years, clinical studies have demonstrated high success rates of up to 98.8 %6. This has been shown in studies both by university- and practice-based groups, with up to 4,591 implants in the follow-up6-10. The implants have also proven successful in various indications, e.g. full-arch rehabilitations in the maxilla11.

How to become an expert
The Straumann® TLX Implant System is built on a strong basis of scientific studies and educational material.
Moreover, in a huge independent study Straumann® implants with an SLA® surface had the lowest odds ratio* for early implant loss, in comparison with two-part bone level implants of other well-documented brands (Figure 3)12. They also had a significantly lower odds ratio for developing peri-implantitis after 9 years, in comparison with bone level implants5. This may be especially relevant in patients with a history of periodontitis.
All of these results underline the high predictability of implant therapy with the Tissue Level concept developed by Straumann®.
* biometric risk measure, applied for the strength of association between two events (factors)
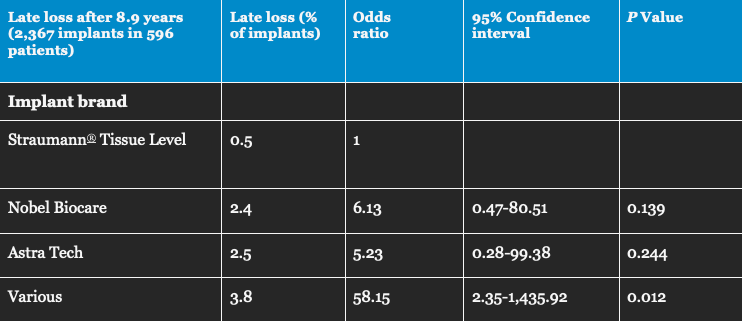
Fig. 3: Percentage and odds ratio (risk measure) for late loss of Straumann® Tissue Level implants (A), in comparison with other well-documented bone level implant brands (B: Nobel Biocare, C: Astra Tech), and various less well-documented brands (D) (12). Based on a total of more than 2,300 implants placed and followed-up in Swedish dental clinics, late loss after 9 years was between 5 and 6 times less likely for Straumann® Tissue Level implants in comparison with bone level implants in groups B and C.

Want to stay up to date?
youTooth.com is THE PLACE TO BE IN DENTISTRY – subscribe now and receive our monthly newsletter on top hot topics from the world of modern dentistry.
