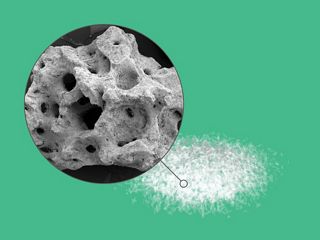
Biphasic calcium phosphate granules.
Based on the knowledge on synthetic biphasic calcium phosphates, maxresorb® comes with a nanostructured surface to provide ideal conditions for the adhesion of osteoblasts. The slow resorption properties facilitate true bone regeneration. 1-5
Features and benefits
The chemical process technology used in the production of maxresorb® ensures high reproducibility and safety of the material. The chemical process technology used in the production of maxresorb® ensures high reproducibility and safety of the 100% synthetic material. 6
maxresorb® exhibits a controlled biphasic resorption; initial integration of the particles followed by complete resorption. While the fast resorption of β-TCP quickly offers space for new bone formation, the HA component provides volume stability for an extended time period. 1,2
maxresorb® is not only a mixture of HA and β-TCP particles. During the production process HA and β-TCP are brought together in a ceramic slurry, which is then foamed and freeze-dried to form particles containing both phases. The fast resorption of the β-TCP component continuously increases the porosity of the material promoting tissue integration by allowing ingrowth of cells and blood vessels. 7,8
The prominent surface roughness of maxresorb® facilitates adhesion of cells and proteins and offers excellent hydrophilicity. maxresorb® is therefore a prominent scaffold for the migration of bone forming cells and binding of signaling molecules from the blood, which lead to accelerated integration and tissue regeneration. 5,9,10
maxresorb® mixes well with autogenous bone, blood or saline solution. It retains liquids facilitating quick and extensive wetting of particles with blood or saline solution. The stickiness of wetted particles allows for quick and easy application to the defect site.
Featured stories on youTooth
Brochures and videos
Looking for additional information? Visit the Resource Center.